The Her-2/Neu (Erbb2) Oncogene In Breast Cancer: Prognostic Factor, Predictive Factor and Target of Therapy
INTRODUCTION
During the past few decades, with the explosion of molecular technology and understanding of the biology of breast cancer, numerous studies have been performed to identify prognostic and predictive factors in breast cancer, with mixed success.
Approximately, 15 percent of all newly diagnosed breast cancers are HER2-positive, meaning that the tumors have extra copies of the HER2 gene and/or high levels of the HER2 protein, which controls Breast Cancer cell growth and spread. HER2-positive tumors usually grow faster compared to HER2-negative tumors.
The purpose of HER2 testing is to identify patients who could benefit from effective HER2-targeted therapies, such as trastuzumab (Herceptin), lapatinib (Tykerb), pertuzumab (Perjeta), and T-DM1 (Kadcyla).
These treatments can substantially improve survival in patients with HER2-positive invasive Breast Cancer. It is therefore important to accurately determine the HER2 status to ensure that patients most likely to benefit are offered a HER2-targeted treatment. At the same time, those that are unlikely to benefit can avoid side effects and costs associated with those drugs.
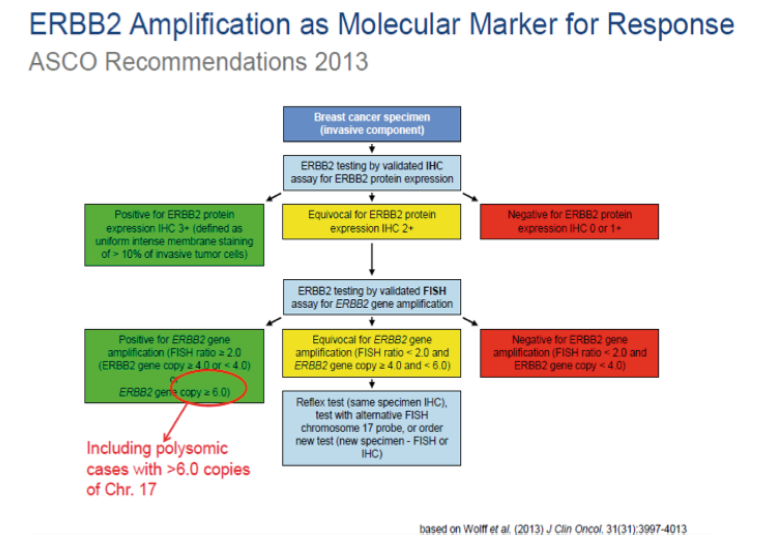
The American Society of Clinical oncology recommends routine measurement of Her-2/neu over-expression and possibly amplification for all patients for breast cancer diagnosis.
The ASCO guideline recommends the following*:
- Always test HER2 status on all newly diagnosed invasive breast cancers (primary site and/or metastatic site). Ensure that at least one tumor sample is tested for either HER2 protein expression (immunohistochemistry [IHC] assay) or (in situ hybridization FISH assay]) for HER2 gene amplification.
- Discuss the role of HER2-targeted therapy if the HER2 test result is positive and if there is no apparent histopathologic discordance with HER2
- Delay the decision to recommend HER2-targeted therapy if the HER2 test result is Mandatory retesting should be done on the same specimen using the alternative test if the initial HER2 test result is equivocal or on an alternative specimen.
- Do not administer HER2-targeted therapy if the HER2 test result is If there is apparent histopathologic discordance with the HER2 test result, additional HER2 testing should be considered.
- Report a HER2 test result as indeterminate if technical issues prevent one or both tests (IHC and ISH) from being done in a tumor specimen, or prevent the test (or tests) from being reported as positive, negative, or
- Confirm that the testing laboratory conforms to standards set for accreditation by CAP or an equivalent accreditation
INDIAN SCENARIO
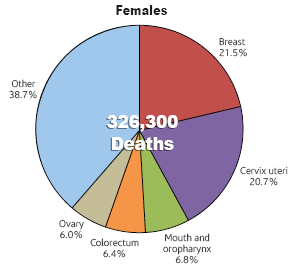
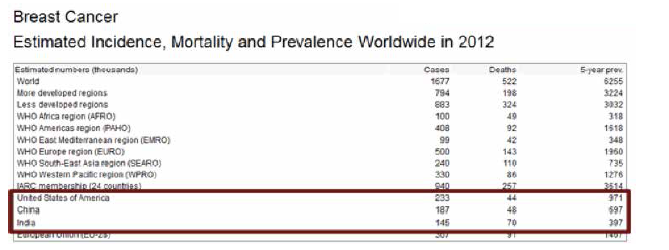
Collectively, US, India and China account for almost one third of the global breast cancer burden.
India accounts for 25% to 31% of all cancers in women in Indian cities. (Source: PBCR 2009 – 2011)
For India in the year 2012:
- 144,937 women were newly detected with breast cancer
- 70,218 women died of breast cancer
- 144937 / 70218 = 06 = round it off to 2. So roughly, in India, for every 2 women newly diagnosed with breast cancer, one lady is dying of it
THE HER-2/neu ONCOGENE
The gene encoding HER2 is located on chromosome 17 and is a member of the EGF/erbB growth factor receptor gene family, which also includes epidermal growth factor receptor (EGFR, or HER1), HER3/erbB3 and HER4/erbB4. All of these genes encode transmembrane growth factor receptors, which are tyrosine kinase type 1 receptors with growth stimulating potential. Activation of HER family members generally occurs when the ligand and a dimer of the same monomer or other member of the HER family are bound together. HER2 has no known ligand. Once activation has occurred, tyrosine autophoshorylation of cytoplasmic signal proteins transmit signals to the nucleus, thus regulating aspects of cell growth, division, differentiation and migration.
Overexpression of HER2 receptors results in receptors transmitting excessive signals for cell proliferation to the nucleus. This may lead to more aggressive growth of the transformed cell. Data supports the hypothesis that the HER2- overexpression cells directly contribute to the pathogenesis and clinical aggressiveness of tumors. This overexpression is associated with poor prognosis, including reduced relapse-free and overall survival.
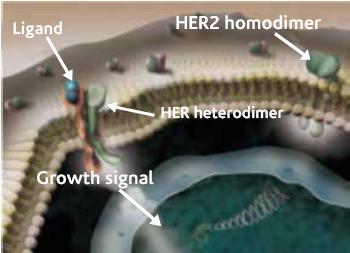 Figure 1 : Representation of HER2 family
Figure 1 : Representation of HER2 family
OTHER USES OF HER-2/neu IN BREAST CANCER
Antibodies to the HER-2/neu protein have been used for radionucleotide imaging in breast cancer. In a 1994 study, Allan and coworkers used Tc99m-labeled monoclonal antibodies to HER-2 protein and reported encouraging results3. In addition to a potential use as a detector of occult metastasis, this approach may also allow for antibody-targeted chemo or radiation therapy in patients with recurrent or metastatic disease.
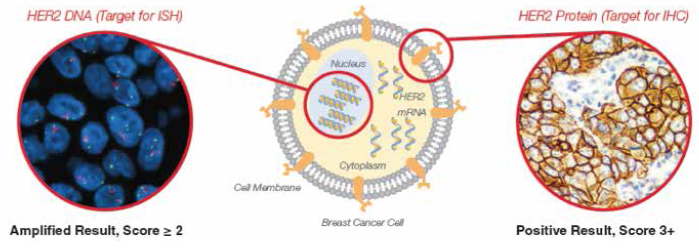
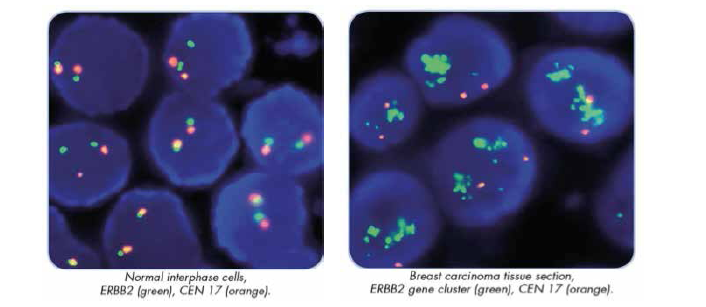
HER-2 NEU TESTING IHC and FISH
Immunohistochemistry (IHC) measures the level of HER2 receptor overexpression, while Fluorescence In-Situ Hybridization (FISH) quantifies the level of HER2 gene amplification. Together they are the most commonly used methods of determining HER2 status in routine diagnostic settings.
 ADVANTAGES OF FLUORESCENCE IN SITU HYBRIDIZATION FOR HER-2/NEU STATUS IN BREAST CANCER
ADVANTAGES OF FLUORESCENCE IN SITU HYBRIDIZATION FOR HER-2/NEU STATUS IN BREAST CANCER
- DNA target is more stable after tissue
- Objective Slide scoring
- Built in internal control (2 gene copies on average demonstrated in non amplified benign and malignant cells).
- Rapid, sensitive, and specific detection and characterization of chromosome abnormalities.
- Ability to test metaphase chromosomes from cultured samples and interphase cells from specimens that cannot be
- Direct-labeled probes, as compared to indirect labeling methods, mean:
-Less background signal, thereby simplifying interpretation.
-Reduced costs associated with labeling reagents and technician time.
- Dual colored probes for most
CONCLUSION
The FISH technique has out-performed a number of solid matrix blotting techniques designed to detect HER-2/neu DNA and RNA as well as immunohistochemistry designed to detect HER-2/neu protein in formalin-fixed paraffin- embedded tissues1. The FISH technique has been described as a rapid, reproducible and extremely reliable method of detecting HER-2/neu gene amplification [48]. In addition, FISH can readily be performed on archived paraffin blocks stored for long periods and has been successfully applied to fine-needle aspiration biopsies2.
SUMMARY
The testing of newly diagnosed Breast Cancer specimens for HER-2 neu status has now achieved standard of practice status for the management of breast cancer.
The discussion as to the best method to determine HER-2 neu status comes to a stop with the FISH Method gaining popularity due the advantages and recent evidence that it in comparison with IHC , may be more accurately predict clinical responses to trastuzumab-based therapies.
However with the other gene based CISH technique waiting in the wings, the story of HER-2/neu testing in breast cancer will continue to unfold over next coming years.
TEST RANGE AVAILABLE FOR BREAST CANCER
REFERENCES
- Press MJ, Bernstein L, Thomas PA et Her-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 1997.
- Sauter G, Feichter G, Torhorst J et Fluorescence in-situ hybridization for detecting ERBB-2 amplification in breast tumor fine needle aspiration biopsies.
- Allan SM, Dean CJ, Eccles S et Clinicoradioimmuno-localization with a rat monoclonal antibody directed against C-erb B2. C
- Slamon DJ, Clark GM, Wong SG et Human breast cancer: correlation of relapse and survival with amplification of the Her-2/neu oncogene. Science 1987;235:177-182.
- Berger MS, Locher GW, Saurer S et Correlation of c-erb B2 gene amplification and protein expression in human breast carcinoma with nodal status and nuclear grading.
- van de Vivjer MJ, Peterse JL, Mooi WJ et Neu-protein overexpression in breast cancer. N Engl J Med 1988;319:1239-1245.
- Heintz NH, Leslie KO, Rogers LA et Amplification of the c-erb B-2 oncogene in prognosis of breast adenocarcinoma.
- Tsuda H, Hirohashi S, Shimosato Y et Correlation between histologic grade of malignancy and copy number of c-erbB-2 gene in breast carcinoma. A retrospective analysis of 176 cases.
- Borg A, Tandon AK, Sigurdsson H et HER-2/neu amplification predicts poor survival in node-positive breast cancer.
- Paik S, Hazan R, Fisher ER et Pathologic findings from the nations’ surgical adjuvant breast and bowel project: prognostic significance of erb B2 protein overexpression in primary breast cancer.
- Battifora H, Gaffey M, Esteban J et Immunohistochemical assay of neu/c-erb B-2 oncogene product in paraffin-embedded tissues in early breast cancer: Retrospective follow-up study of 245 stage I and II cases.
- Kallioniemi OP, Holli K, Visakorpi T et Association of Cerb B2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer.
- Clark GM, McGuire Follow-up study of HER-2/neu amplification in primary breast cancer.
- Lovekin C, Ellis IO, Locker A et C-erb B2 oncoprotein expression in primary and advanced breast cancer.
- McCann AH, DeDervan TA, O’Regan M et Prognostic significance of C-erb B2 and estrogen receptor status in humanbreast cancer.